Carbon dioxide.
Carbon dioxide is a chemical elements. Human chemical develop fully depends on it. Let’s know briefly about it.
What is Carbon dioxide?
Carbon dioxide is an acidic colorless and odorless gas at atmospheric temperatures and pressures. It has a faint sharp odor and a sour taste. It is one of the most important greenhouse gases which is the main reason for global warming. On the other side, it is one of the minor component of earth’s atmosphere formed in combustion of carbon containing materials.
Discovery of carbon dioxide.
In the early 17th century, the Flemish chemist Jan Baptist Van Helmont observed that when he burned charcoal in a closed vessel.
The properties of carbon dioxide were further studied in the 1750s by the Scottish physician Joseph Black. He found that limestone could be heated with acids to yield as gas.
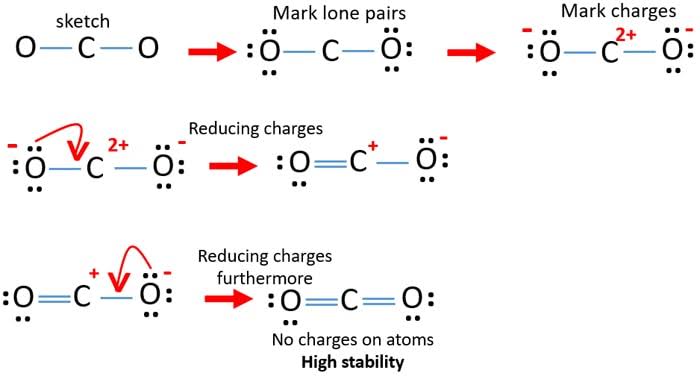
Physical and chemical properties of carbon dioxide.
At normal temperature, carbon dioxide is quite unreactive. Above 1700oC, it partially decomposes into carbon mono-oxide and oxygen. Hydrogen or carbon also convert it to carbon mono-oxide at high temperature.
It is slightly soluble in water forming a weakly acidic solution. This solution contains the dibasic acid called carbonic acid.
At standard temperature and pressure, the density of carbon dioxide is around 1.98 kg/m3, which is about 1.53 times that of air.
It has no liquid state below the pressure 5.111 77(99) atm.
The symmetry of a carbon dioxide molecule is linear and centrosymmetric at its equilibrium geometry. The length of the carbon–oxygen bond in carbon dioxide is 116.3 pm.
In the gas phase, carbon dioxide molecules undergo significant vibrational motions and do not keep a fixed structure.
In aqueous solution, carbon dioxide is soluble in water, in which it reversibly forms H2CO3, which is a weak acid.
CO2 is a potent electrophile having an electrophilic reactivity that is comparable to benzaldehyde. In metal carbon dioxide complexes CO2 serves as a ligand, which can facilitate the conversion of CO2 to other chemicals.
Production and uses of Carbon dioxide.
Carbon dioxide can be obtained by distillation from air. The production of all carbon based fuels such as- methane, gasoline, diesel, kerosene, propane, coal, wood etc. produces carbon dioxide.
Carbon dioxide is a byproduct of the industrial production of hydrogen by steam reforming and the water gas shift reaction in ammonia production. This is a major source of food grade carbon dioxide for the uses in carbonation of bear and soft drinks.
All the aerobic organisms produce CO2 when they oxidize carbohydrates, fatty acids and proteins.
Aerobic organisms decompose organic material producing methane and carbon dioxide together with traces of other compounds.
It is used as a refrigerant, in fire extinguishers for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics etc.
It is used in different food industries, oil industries and the chemical industries.
Carbon dioxide is largely used in agricultural sector. Plants require Carbon dioxide to conduct the photosynthesis process.
Again, it is also used as a propellant and acidity regulator in the food industry. Bakers’ yeast produces Carbon dioxide by fermentation of sugars within the dough, while chemical liveners such as baking powder and baking soda release Carbon dioxide when heated.
Again, it is also used in the production of beverages like carbonated soft drinks and soda water.
Another most important uses of Carbon dioxide are its uses in the security for fire extinguishers occurrence. It can be used to extinguish flames by flooding the environment around the flame with the gas.
In the medical sector, it is used up to 5% Carbon dioxide adding to oxygen for stimulation of breathing.
Carbon dioxide and environment.
Carbon dioxide is the most important of earth’s long lived greenhouse gases. It absorbs less heat per molecule than the other greenhouse gases methane or nitrous oxide.
Increasing carbon dioxide in the atmosphere is responsible for about two-thirds of the total energy imbalance that is the main reason for the temperature rise on the earth.
Extra carbon dioxide in the atmosphere increases the greenhouse effect. More thermal energy is trapped in the atmosphere. As a result, the planet is becoming warmer than it would be naturally.
More added that,
By increasing temperature and humidity, carbon dioxide emissions increase the formation of smog, which has an adverse effect in respiratory health of living beings.
Frequently Asked Questions (FAQ).
1. What is Carbon dioxide?
Answer: Carbon dioxide is an acidic colorless and odorless gas at atmospheric temperatures and pressures. It has a faint sharp odor and a sour taste. It is one of the most important greenhouse gases which is the main reason for global warming. On the other side, it is one of the minor component of earth’s atmosphere formed in combustion of carbon containing materials.
2. What is carbon dioxide used for?
Answer: It is used as a refrigerant, in fire extinguishers for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics etc.
3. Is carbon dioxide necessary for human beings?
Answer: Although, carbon dioxide has some harmful effects, in spite of that, it is an important greenhouse gas that helps to trap heat in our atmosphere. Without it, our planet will become adverse for our livings.
4. How is carbon dioxide created?
Answer: When hydro-carbon fuels like wood, coal, natural gas, gasoline and oil are burned, carbon from fossil fuels combine with oxygen in the air to form carbon dioxide and water vapor. But now a day, carbon dioxide is being added to the atmosphere by human activities.
5. How is trees and plants are inter-related with human beings?
Answer: Living beings mostly humans, while respiration, they intake oxygen and releases carbon dioxide in the atmosphere. This released carbon dioxide is taken by the trees and plants during their photosynthesis process. Thus, the environment is balanced.
How much you learn? Drop a comment. Stay with us.